Chapter Five (A) — Results and Statistics Analysis.
Preface
THIS WILL BE PUBLISHED PER CHAPTERS: PART 5/8.
During my final year of my bachelor of pharmacy, I had the joy of doing a project in pharmaceutics on topical corticosteroids. My colleague and I achieved a distinction for this project but we do admit that we can see some mistakes in our project. This project took us 6 months to accomplish and it mainly taught us about research, reading the literature and to attempt at making a pharmaceutics project. This was more of an introduction to a masters degree path and also taught us to work on our own and be supervised with an allocated supervisor. All in all, we learned a great deal and we were thankful for it.
Authors: Andréas L.P. Astier, Priyanka Naidoo
Supervisors: Professor R. Walker, Dr S.M. Khamanga
Location: Pharmaceutics Department, Rhodes University, Grahamstown, 6140, South Africa.
CHAPTER FIVE
Results and Statistics Analysis
5.1. Introduction
The collecting of the results expanded over a 28-day period where 4 dissolutions curves were made. On Day 60 the last dissolution curve was made to expand on the degradation studies
5.2. Calibration curve construction
A constant stock solution was used throughout the whole experiment.
Stock concentration: 0,1 grams (100 mg):
1 litre (1000 mL)
100 μg : 1 mL -> 100 μg/mL
Different concentrations, up to five, were used. These were made the following way respectively of their concentration:
Example: 40 μg/mL, 100 mL volumetric flask were used :
C1V1 = C2V2
(100) (V1) = (40) (100)
V1 = 40ml
An average was used for each point to plot the calibration curve. Each point had a standard deviation. Only two calibrations were used, and the average of these two was calculated and used throughout al the dissolutions curves as a standard as it best described the relationship between the absorbance and the concentration (μg/mL). The linear regression line calculates an equation that minimizes the distance between the fitted line and all of the data points. This was automatically calculated by Microsoft Excel 2016 program. An r2 was also available and calculated.
5.2.1. Calibration curve data on Day 0, 7, 14, and 28 with the calculated standard
Calibration curve on Day 0, 7, 14 and 28.
Average calibration curve between Day 14 and 28.






Concentrations points with the amount of stock solution and deionised water on Day 0, 7, 14 and 28.
Concentration points and its average absorbance on Day 0, 7, 14 and 28.




5.2.2. Standard calibration curve
The average between the calibration curve of day 14 and 28 was chosen as a standard for every dissolution curve. The reason was that the CBP was highly non-dissolvable in an aqueous medium, hence the calibration curve of day 14 and 28 best represented the relationship between the absorbance and the concentration (μg/mL). This is due to the CBP being sonicated numerous times and left to be dissolved in the deionised water for a long period of time. The CBP stability was unaltered as mentioned in the literature papers of CBP stability (26, 27) and discussed in section 1.3.2.
Standard equation of the linear regression line: y = 0,0109x - 0,0248 R2 = 0,99908
The r2 value tells us how close the data is to the fitted regression line (66, 67). An r2 value of 0.0 means that knowing the absorbance does not help to predict the concentration (μg/mL) (66, 67). When r2 equals 1.0, all points lie exactly on a straight line with no scatter. 0% indicates that the model explains none of the variability of the response data around its mean (66, 67). 100% indicates that the model explains all the variability of the response data around its mean. Therefore, the higher the r2 value, the better the model fits the data (66, 67). In this instance, the r2, 0.99908 (99,908%), value is close to 1 (or 100%) hence it means that our model fits the data precisely and accordingly and that we are confident that our data properly depict a reliable relationship between the absorbance and the concentration (μg/mL).
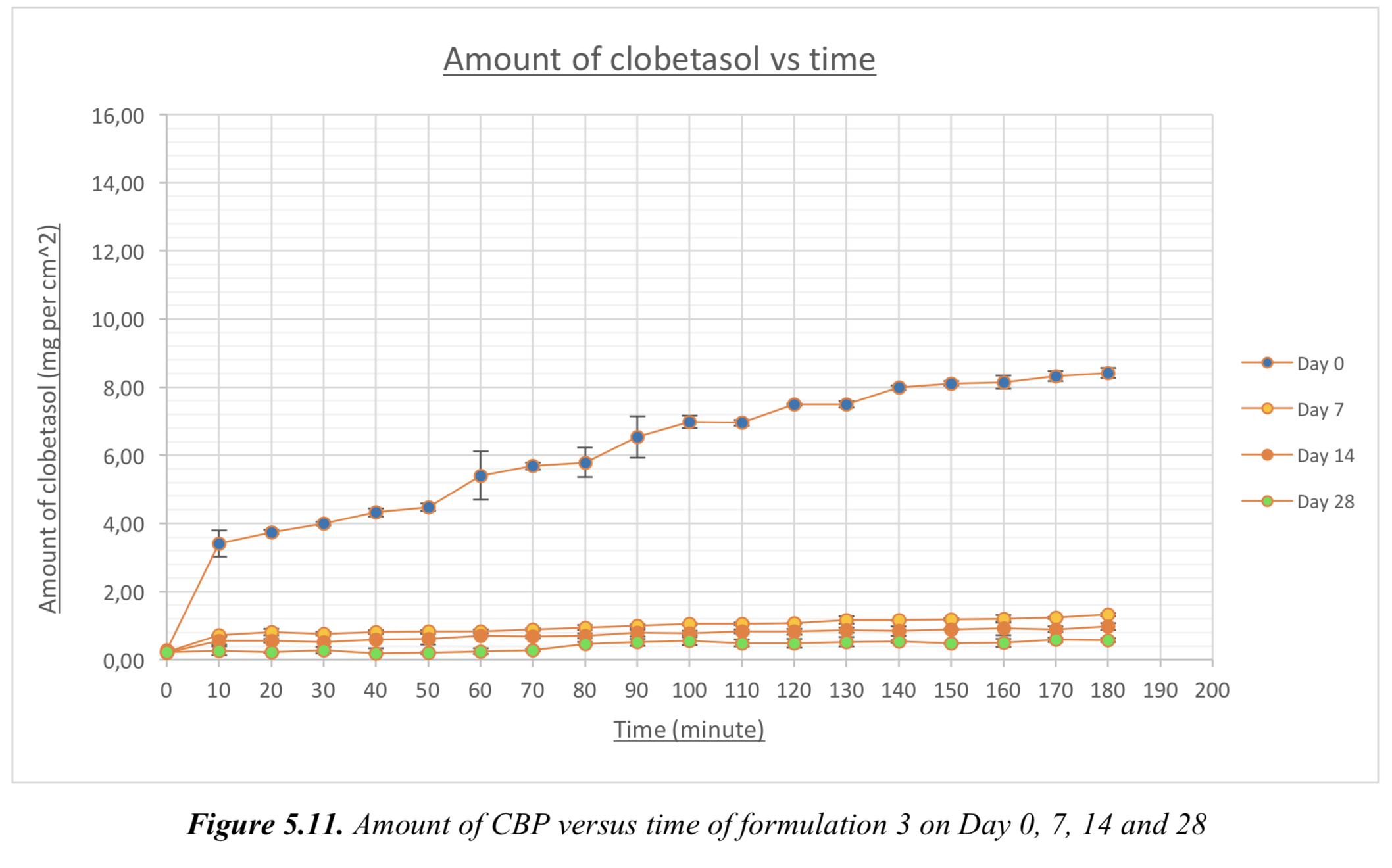
5.3. Dissolution data of the formulations on Day 0, 7, 14, 28 and 60
Amount of CBP versus time of formulation 1, 2, 3 and 4 on Day 0, 7, 14, 28 and 60.








Percentage of CBP versus time of formulation 1, 2, 3 and 4 on Day 0, 7, 14, 28 and 60.









5.4. Spreadability data of the formulations on Day 0, 7, 14 and 28
Two glass plates were used, with 2 grams of the allocated formulation placed at the centre. Two 100 grams’ weight was placed on top for exactly 5 minutes. The distance was measured, and an average and standard deviation were calculated.
Spreadability data of formulation 1, 2, 3 and 4 on Day 0, 7, 14 and 28.
5.5. Degradation studies with theoretical values
Formulation 1, 2, 3 and 4 average percentage plateau release with its respective time in days.
Dissolution curve made on the Friday 7th of October 2016, Day 60 since the manufacture of the creams. These will determine if the theoretical value of predicting the average plateau release to be significantly accurate.


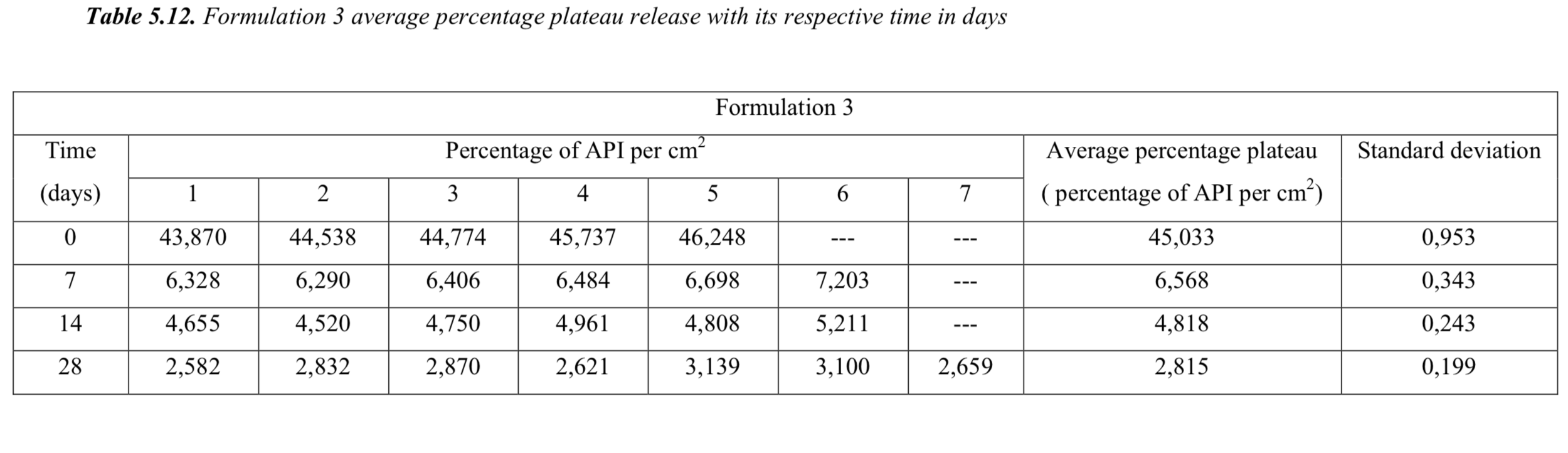


Figure 5.21. Linear line equation on a semi-log graph paper of formulation 1, 2, 3 and 4.




5.6. Accumulation studies
Patients are required to use no more than 50 grams of cream per week (28, 29). That is 3,571 grams for each use twice a day (4, 16, 20). The accumulation study shows how much a patient actually needs to use to cause the wanted therapeutic effect. Each formulation will differ depending on their average plateau release from their respective dissolution curve. These data are plotted on a graph and the area under the curve (AUC) was calculated. A red arrow will then show the time (days) when a patient would have run out of cream if they had a 200 gram CPB aqueous cream. However, we have no idea how much and how consistent patients spread their CBP aqueous cream. The severity of the disease, age, sex, race, gender, surface area are factors to consider. All usage should be within 2 weeks.




Published 4th August 2019. Last reviewed 30th December 2021.
Reference
4. Division of Clinical Pharmacology Faculty of Health Sciences, 2014. South African Medicines Formulary, 11th Ed., Cape Town, South Africa.
16. Division of Clinical Pharmacology Faculty of Health Sciences, 2016. South African Medicines Formulary, 12th Ed., Cape Town, South Africa.
20. PubChem, 2016. Clobetasol Propionate. [Online] Available at: https://pubchem.ncbi.nlm.nih.gov/compound/clobetasol_propionate#section=Computed-Properties [Accessed 21st September].
26. Mohammad, S.A., Mohammad A., Nawazish, A., Tarique, A., Mohammed, A.S. Accelerated Stability Testing of a Clobetasol Propionate-Loaded Nanoemulsion as per ICH Guidelines. Journal of Separation Science, 81 (2013) 1089-1100.
27. Fauzee, A.F., Walker, R.B. Forced degradation studies of clobetasol 17-propionate in methanol, propylene glycol, as bulk drug and cream formulations by RP-HPLC. Journal of Separation Science, 36 (2013) 849-856.
28. WebMed, 2016. Clobetasol Topical. [Online] Available at: http://www.webmd.com/drugs/2/drug-4403-723/clobetasol-topical/clobetasol---topical/details [Accessed 21st September].
29. Drugs, 2016. Clobetasol Cream. [Online] Available at: https://www.drugs.com/pro/clobetasol-cream.html [Accessed 21st September].
66. Duke, 2016. Statistical forecasting: notes on regression and time series analysis. [Online] Available at: http://people.duke.edu/~rnau/rsquared.htm [Accessed 26th September].
67. The Minitab Blog, 2016. Regression Analysis: How Do I Interpret R-squared and Assess the Goodness-of-Fit. [Online] Available at: http://blog.minitab.com/blog/adventures-in-statistics/regression-analysis-how-do-i-interpret-r-squared-and-assess-the-goodness-of-fit [Accessed 26th September].